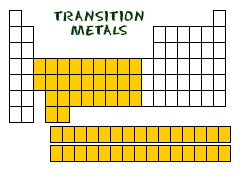
The 38 elements in groups 3 through 12 of the periodic table are called “transition metals”. As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat. The interesting thing about transition metals is that their valence electrons, or the electrons they use to combine with other elements, are present in more than one shell. This is the reason why they often exhibit several common oxidation states. There are three noteworthy elements in the transition metals family. These elements are iron, cobalt, and nickel, and they are the only elements known to produce a magnetic field. Each group in the transition metals has complete s and p orbitals with incomplete d orbitals. The elements tend to want the most stable configuration; for example, one electron in each orbital instead of a complete s orbital and four d orbitals with one electron each. This leads to some unique characteristics.
The transition elements often act as catalysts in reactions and are often colorful in compounds.
The Transition Metals are:
| Scandium (Sc) | Yttrium (Y) | Lutetium (Lu) | Lawrencium (Lr) |
| Titanium (Ti) | Zirconium (Zr) | Hafnium (Hf) | Rutherfordium (Rf) |
| Vanadium (Va) | Molybdenum | Tantalum (Ta) | Dubnium (Db) |
| Chromium (Cr) | Technetium (Tc) | Tungsten | Seaborgium (Sg) |
| Manganese | Ruthenium (Ru) | Rhenium(Re) | Bohrium (Bh) |
| Iron (Fe) | Rhodium (Rh) | Osmium (Os) | Hassium (Hs) |
| Cobalt (Co) | Palladium (Pd) | Iridium (Ir) | Meitnerium (Mt) |
| Nickel (Ni) | Silver (Ag) | Platinum (Pt) | Damstadium(Uun) |
| Copper (Cu) | Lutetium (Lu) | Gold (Au) | Unununuim (Uuu) |
| Zinc (Zn) | Cadmium (Cd) | Mercury (Hg) | Ununbium (Uub) |

Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. Think about argon (Ar). It has 18 electrons set up in a 2-8-8 order. Scandium (Sc) is only 3 spots away with 21 electrons, but it has a configuration of 2-8-9-2. Wow! This is where it starts. This is the point in the periodic table where you can place more than 8 electrons in a shell. You need to remember that those electrons are added to the second-to-last shells. The transition metals are able to put up to 32 electrons in their second-to-last shell. Something like gold (Au), with an atomic number of 79, has an organization of 2-8-18-32-18-1. Of course, there are still some rules. No shell can have more than 32 electrons. You will find it’s usually 2, 8, 18 or 32 for the maximum number of electrons in an orbital.
Most elements can only use electrons from their outer orbital to bond with other elements. Transition metals can use the two outermost shells/orbitals to bond with other elements. It’s a chemical trait that allows them to bond with many elements in a variety of shapes. Why can they do that? As you learn more, you will discover that most transition elements actually have two shells that are not happy. Whenever you have a shell that is not happy, the electrons want to bond with other elements. Example: Molybdenum (Mo), with 42 electrons. The configuration is 2-8-18-13-1. The shells with 13 and 1 are not happy. Those two orbitals can use the electrons to bond with other atoms.