Metalloids are the elements found along the stair-step line that distinguishes metals from non-metals. This line is drawn from between Boron and Aluminum to the border between Polonium and Astatine. The only exception to this is Aluminum, which is classified under “Other Metals”. Metalloids have properties of both metals and non-metals. Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators. The metalloids are a group of elements in the periodic table. They are located to the right of the post-transition metals and to the left of the non-metals. Metalloids have some properties in common with metals and some in common with non-metals. They appear to be metal in appearance, but are brittle. They can generally form alloys with metals. Some metalloids such as silicon and germanium become electrical conductors under special conditions. These are called semiconductors. They are solids under standard conditions. They are mostly nonmetallic in their chemical behavior.
The Metalloids are:
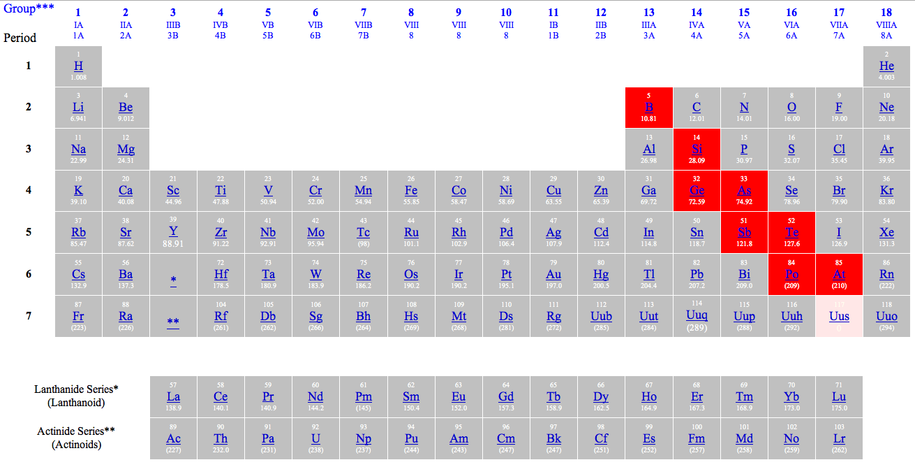
- Boron (B)
- Silicon (Si)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
- Polonium (Po)
Boron (B)

Name: Boron
Symbol: B
Atomic Number: 5
Atomic Mass: 10.811 amu
Melting Point: 2300.0 °C
Boiling Point: 2550.0 °C
Number of Protons/Electrons: 5
Number of Neutrons: 6
Classification: Metalloids
Crystal Structure: Rhombohedral
Density @ 293 K: 2.34 g/cm3
Color: brownish
Silicon (Si)

Name: Silicon
Symbol: Si
Atomic Number: 14
Atomic Mass: 28.0855 amu
Melting Point: 1410.0 °C
Boiling Point: 2355.0 °C
Number of Protons/Electrons: 14
Number of Neutrons: 14
Classification: Metalloids
Crystal Structure: Cubic
Density @ 293 K: 2.329 g/cm3
Color: grey