Chemistry is the scientific discipline involved with elements and compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. It is the branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances. Chemistry is centrally concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds.
History of Chemistry
In many ways, the history of civilization is the history of chemistry — the study of matter and its properties. Humans have always sought to identify, use and change the materials in our environment. Early potters found beautiful glazes to decorate and preserve their wares. Herdsmen, brewers and vintners used fermentation techniques to make cheese, beer and wine. Housewives leached the lye from wood ash to make soap. Smiths learned to combine copper and tin to make bronze. Crafters learned to make glass; leatherworkers tanned hides. In the eighth century A.D., Jābir ibn Hayyān, a Muslim astronomer, philosopher and scientist, became one of the first to use scientific methods to study materials. Also known by his Latinized name, Geber, he is known as the “father of chemistry.” He is thought to be the author of 22 scrolls describing methods of distillation, crystallization, sublimation and evaporation. He invented the alembic, a device used to distill and study acids. He also developed an early chemical classification system using the properties of the materials he studied. Robert Boyle studied the behavior of gases and discovered the inverse relationship between volume and pressure of a gas. He also stated that “all reality and change can be described in terms of elementary particles and their motion,” an early understanding of atomic theory. In 1661, he wrote the first chemistry textbook, “The Sceptical Cymist,” which moved the study of substances away from mystical associations with alchemy and toward scientific investigation. Dmitri Mendeleev was a Russian chemist known for developing the first Periodic Table of Elements. He listed the 63 known elements and their properties on cards. When he arranged the elements in order of increasing atomic mass, he could group elements with similar properties. With a few exceptions, every seventh element had similar properties. Mendeleev realized that if he left spaces for the places where no known element fit into the pattern that it was even more exact. Using the blank spaces in his table, he was able to predict the properties of elements that had yet to be discovered. Mendeleev’s original table has been updated to include the 92 naturally occurring elements and 26 synthesized elements. In 1935, James Chadwick was awarded the Nobel Prize for his discovery that there are an equal number of electrically neutral particles in the nucleus of an atom. Since neutrons are electrically neutral, they are not deflected by either electrons or protons. Furthermore, neutrons have more mass than protons. These facts combine to make it possible for neutrons to penetrate atoms and break apart the nucleus, releasing vast amounts of energy.
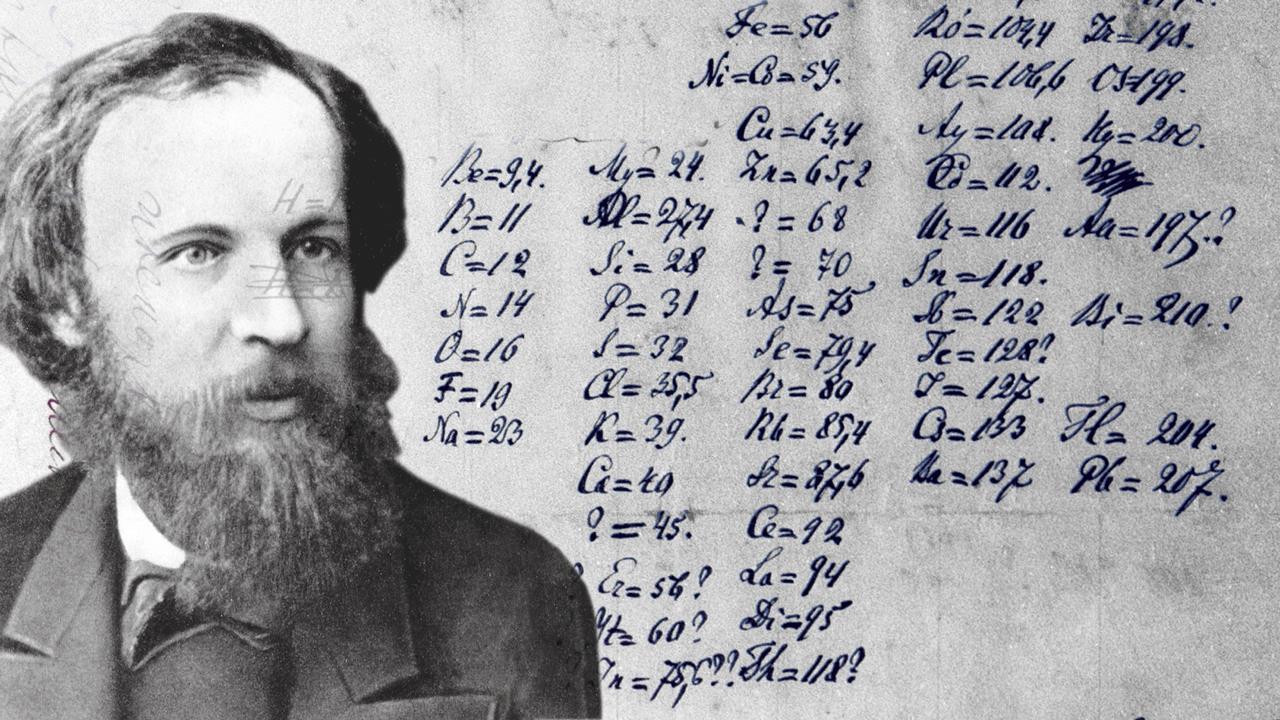
Chemistry Branches
- Organic Chemistry : The study of carbon and its compounds; the study of the chemistry of life
- Inorganic Chemistry: The study of compounds not covered by organic chemistry; the study of inorganic compounds, or compounds that don’t contain a C-H bond
- Analytic Chemistry: The study of the chemistry of matter and the development of tools to measure properties of matter
- Physical Chemistry: The branch of chemistry that applies physics to the study of chemistry, which commonly includes the applications of thermodynamics and quantum mechanics to chemistry
- Biochemistry: The study of chemical processes that occur inside of living organisms